DePuy bags FDA 510(K) clarence for robotic-assisted knee surgery device
The clearance for unicompartmental knee arthroplasty follows a previous clearance for the device in total knee arthroplasty.The post DePuy bags FDA 510(K) clarence for robotic-assisted knee surgery device appeared first on Medical Device Network.
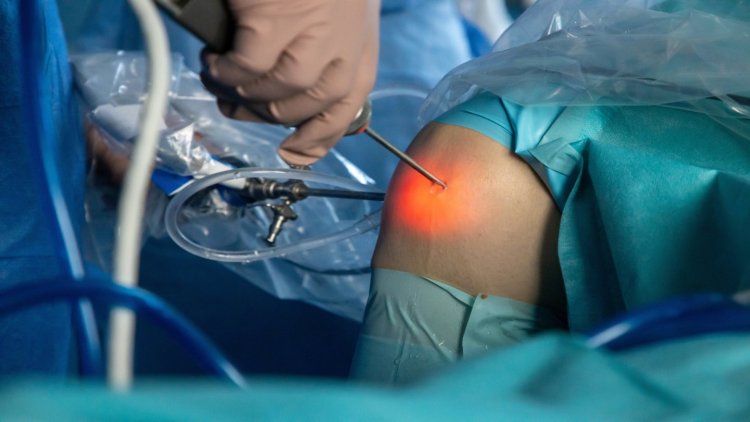
The post DePuy bags FDA 510(K) clarence for robotic-assisted knee surgery device appeared first on Medical Device Network.
What's Your Reaction?












![[Computex] The new be quiet cooling!](https://technetspot.com/uploads/images/202406/image_100x75_6664d1b926e0f.jpg)








